Adaptive deep brain stimulation for Parkinson's disease
June 5, 2018
Adaptive deep brain stimulation for Parkinson's disease
At a Glance
- Researchers tested a self-tuning deep-brain stimulation device in two patients with Parkinson's disease.
- An adaptive system may limit the adverse effects of traditional deep brain stimulation, but considerable testing remains to be done.
Parkinson's disease is a motor system disorder that results from the loss of dopamine-producing brain cells. It causes tremors (trembling in hands, arms, legs, jaw, and face), rigidity (stiffness of the limbs and trunk), bradykinesia (or slowness of movement), and balance and coordination problems.
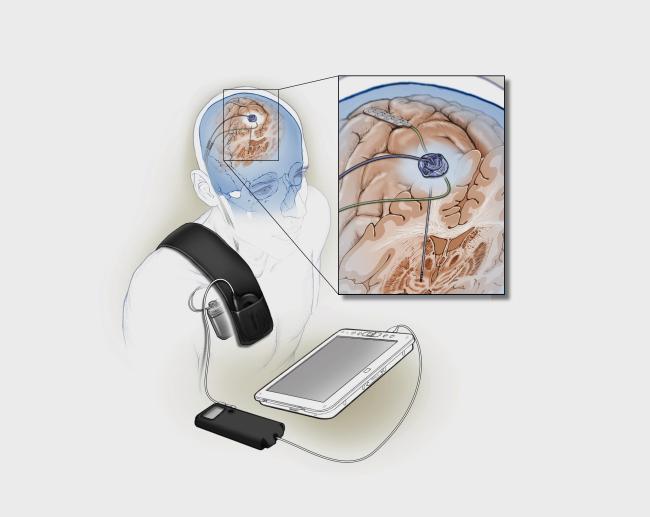
Deep brain stimulation (DBS) has been used to help manage tremors and other Parkinson's disease symptoms for 25 years. Traditional deep brain stimulation delivers constant electrical stimulation to the brain regions that control movement through a surgically implanted thin wire, or electrode.
However, this approach can lead to unwanted side effects. Finding the correct level of stimulation is like trying to hit a constantly moving target. Too much stimulation can cause dyskinesia, or uncontrolled movements. Adjustments to the pulse generator also require reprogramming by a trained clinician. These limitations have led researchers to look for ways to improve the technique.
A team led by Dr. Philip A. Starr at the University of California, San Francisco, tested the first fully implanted DBS system that uses feedback from the brain itself to fine-tune its signaling. The prototype was built in a public-private partnership with Medtronic. The device differs from traditional DBS systems in that it can both monitor and modulate brain activity. The researchers implanted the device into two patients with Parkinson's disease who had traditional DBS but continued to experience dyskinesia after adjustment by a neurologist. The research was supported in part by NIH's BRAIN Initiative and National Institute of Neurological Disorders and Stroke (NINDS). Results were published in the Journal of Neural Engineering on May 9, 2018.
In a previous study, the researchers identified a distinctive signal in the human motor cortex (a part of the brain critical for normal movement) that occurs during dyskinesia. In the new study, they trained a computer program to recognize this pattern. They then programmed an adaptive system to reduce stimulation when it identified the dyskinesia-related brain activity and increase it when there was no dyskinesia. The team compared the results of this adaptive stimulation system with traditional, constant stimulation set manually on the same two patients.
The researchers found that the adaptive approach was as effective at controlling symptoms as constant stimulation. There were no observed or reported differences in movement improvement. Because adaptive deep brain stimulation doesn't continuously stimulate the brain, the system saved about 40% of the device's battery energy from traditional stimulation. The team was unable to compare the incidence of dyskinesia in this feasibility study due to the short time period. They still need to compare the DBS methods over longer time periods to determine whether the adaptive technique can reduce this side effect.
"Here we have demonstrated the feasibility of adaptive deep brain stimulation," Starr says. "We are now planning larger, longer-term trials to determine how effective this system is in managing the symptoms of patients with Parkinson's disease."


