Bacteria therapy improves eczema in children
September 22, 2020
Bacteria therapy improves eczema in children
At a Glance
- An experimental probiotic treatment for eczema safely improved symptoms and increased quality of life for children 3 to 16 years of age.
- The findings support a larger trial to test the safety and effectiveness of the therapy.
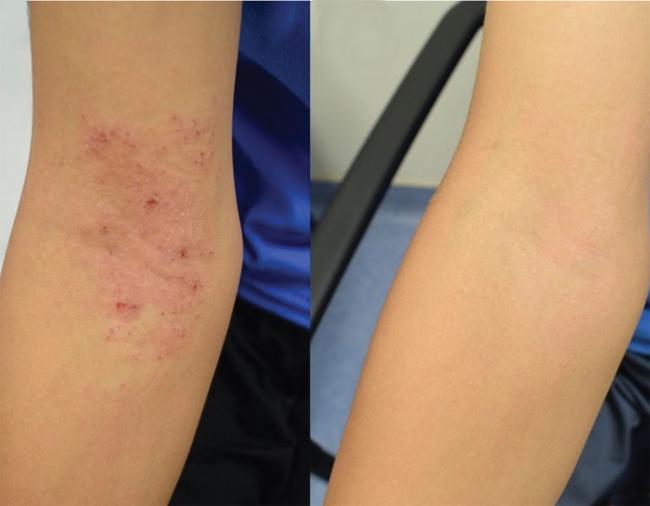
Atopic dermatitis, or eczema, is an inflammatory skin disease. It can cause dry, itchy skin and rashes. It’s also linked to increased risk for asthma, hay fever, and food allergies.
Eczema is most common in children. Many outgrow eczema, but some continue to have symptoms as teens and adults. Medications and skin treatments can help manage eczema symptoms. However, they may be costly and often need to be applied multiple times each day.
The causes of eczema are unclear. Research suggests that the skin’s microbiome—the community of bacteria and other microbes living on the skin—plays a key role. Studies have shown that people with eczema often have a different balance of bacteria than those with healthy skin. Researchers have been testing whether beneficial bacteria might help treat eczema.
Results in mice and cell models suggested that the bacterium Roseomonas mucosa, taken from healthy human skin, could improve eczema. Researchers led by Dr. Ian A. Myles of NIH’s National Institute of Allergy and Infectious Diseases (NIAID) launched a clinical trial to test R. mucosa therapy in people with eczema. In 2018, the team published positive results in 10 adults and five children.
To test whether R. mucosa therapy might benefit younger children, who are most likely to have eczema, the team expanded the study by enrolling 15 additional children, some as young as 3 years old. Findings appeared in Science Translational Medicine on September 9, 2020.
For four months, children or their caregivers sprayed a solution containing live R. mucosa onto areas of skin with eczema. The solution was applied twice weekly for three months and then every other day for an additional month. The researchers gradually increased the dose of live R. mucosa for most of the children enrolled in the study.
Seventeen of the 20 children had a greater than 50% improvement in their eczema symptoms following treatment. The scientists also found increases in the skin’s barrier function—its ability to seal in moisture and keep out allergens. Most children needed fewer corticosteroids to manage their eczema, experienced less itching, and reported a better quality of life following the therapy. These benefits continued after finishing treatment, and R. mucosa strains remained on the skin for up to eight months.
The researchers also studied how R. mucosa therapy improves eczema symptoms. They found that treated skin had more microbial diversity and reduced levels of Staphylococcus aureus, a bacterium known to worsen eczema.
The skin of people with eczema is usually deficient in certain lipids, or oils. The scientists studied a specific class of lipids produced by R. mucosa strains taken from healthy skin. Using cell cultures and mouse models of eczema, they found that these lipids can induce skin repair and promote turnover of skin tissue. Children in the study had increased levels of these lipids on their skin after treatment with R. mucosa.
“Most children in the study experienced substantial improvements in their skin and overall wellbeing following R. mucosa therapy. Encouragingly, the therapeutic bacteria stayed on the skin and continued to provide benefit after therapy stopped,” Myles says. “These results support a larger study to further assess the safety and effectiveness of this experimental treatment by comparing it with a placebo.”
Related Links
- Bacteria Therapy Tested for Common Skin Disease
- Skin Microbes Fairly Stable Over Time
- Probiotic Prevents Infant Infections in Rural India
- The Healthy Human Microbiome
- Rash Decisions
- Eczema (Atopic Dermatitis)


