"Intelligent" injection device targets tissues
March 12, 2019
"Intelligent" injection device targets tissues
At a Glance
- Researchers developed a resistance-sensitive injection device that delivered liquid and cells to a specific area at the back of the eye in animal models.
- The mechanical device would be a cost-effective way to help reduce errors when targeting tissues that are difficult to reach using traditional syringes.
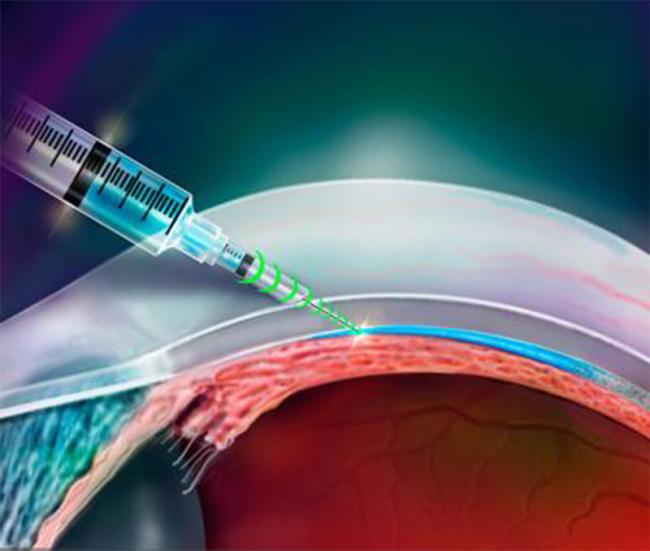
Using a syringe and needle to inject medicines or other liquids into body tissue requires skill and precision. It relies on training and experience to know how deep to push in the needle. Missing the target area can make a medicine less effective and create serious complications.
To explore ways to make needle injections more precise, a team led by Dr. Jeff Karp at Brigham and Women's Hospital developed a resistance-sensing injector device. They tested its ability to deliver drugs and live cells to different tissue at the back of the eye. Certain diseases of the eye could benefit from drug delivery to this area. However, it's very difficult to reliably inject drugs into the thin tissues at the back of the eye.
Using a standard hypodermic needle and pieces of commercially available syringes, the team designed an intelligent injector for tissue targeting (I2T2). I2T2 senses pressure changes in tissue and automatically releases its contents upon a drop in resistance. The work was supported in part by NIH's National Heart, Lung, and Blood Institute (NHLBI). Results were published online on February 25, 2019, in Nature Biomedical Engineering.
The researchers first tested the forces applied by the device and the needle's resistance-feedback system using a universal testing machine. They then tested its ability to inject liquids into tissue in the back of the eye. They injected a green dye, aiming for the suprachoroidal space (SCS), which sits in between the millimeter-thin sclera (the white outer layer of the eye) and the choroid (the layer that supplies blood vessels to the light-sensing retina at the back of the eye). The needle penetrated the sclera and delivered the dye directly into the SCS without going further into the choroid or retina.
After testing the device with dye, the team then tried injecting drug-delivery particles and live cells into the SCS. Using tissue microscopy, they confirmed that the particles and cells reached the SCS. Further, they found that 99% of the live cells survived injections with the device. The injections caused minimal damage to the eyes. These findings suggest the potential of using the device to treat eye conditions in the future.
The researchers also demonstrated that they could use the device to deliver fluid into the spinal canal and the abdomen.
"Targeting specific tissues using a conventional needle can be difficult and often requires a highly trained individual," Karp says. "In the past century there has been minimal innovation to the needle itself, and we saw this as an opportunity to develop better, more accurate devices. We sought to achieve improved tissue targeting while keeping the design as simple as possible for ease of use."
"This intelligent injector is a simple solution that could be rapidly advanced to patients to help increase target tissue precision and decrease overshoot injuries," says researcher Dr. Girish Chitnis. "We have completely transformed needles with a small modification that achieves better tissue targeting."
Further studies are needed to test the device's safety and effectiveness in pre-clinical models and people.
€”by Tianna Hicklin, Ph.D.
References
A resistance-sensing mechanical injector for the precise delivery of liquids to target tissue. Girish D. Chitnis, Mohan K. S. Verma, Julien Lamazouade, Miguel Gonzalez-Andrades, Kisuk Yang, Ali Dergham, Peter Anthony Jones, Benjamin E. Mead, Andrea Cruzat, Zhixiang Tong, Keir Martyn, Aniruddh Solanki, Natalie Landon-Brace & Jeffrey M. Karp. Nature Biomedical Engineering. (2019) 25 Feb; doi.org/10.1038/s41551-019-0350-2.
Funding
NIH's National Heart, Lung, and Blood Institute (NHLBI) and Boston-KPro.


