Lab-grown eye cells form new neural connections
January 24, 2023
Lab-grown eye cells form new neural connections
At a Glance
- Eye cells that were grown from skin-derived stem cells could be collected from 3D cultures and coaxed to form new neural connections.
- These results highlight the potential for using transplanted cells to help restore vision.
In degenerative eye diseases, such as retinitis pigmentosa, macular degeneration, and glaucoma, cells in the eye start to die. These cells capture light or send visual information to the brain. Their loss can eventually lead to reduced vision or even blindness.
Once eye cells die, the body can’t regenerate them. Researchers have been studying whether replacement eye cells could be grown in the lab and transplanted into the eyes to restore sight. For such a strategy to work, the replacement cells would need to be able to form communication links, called synapses, with existing cells.
A research team led by Dr. David Gamm from the University of Wisconsin, Madison has been experimenting with growing human eye cells in culture. In previous work, they showed that stem cells derived from human skin cells could be reprogrammed to produce structures called retinal organoids. The organoids contain many types of cells that the eyes need to see. These include photoreceptors—rods and cones, which sense light—and retinal ganglion cells, which send information to the brain.
To restore vision, cultured cells transplanted into the eye would need to form new synapses. But whether the organoid cells could be harvested and create new synapses hasn’t been shown.
In a new study, Gamm and his colleagues tested this question using a tracer molecule that can travel through connections made by individual neurons. Their results were published on January 10, 2023, in the Proceedings of the National Academy of Sciences.
The team first grew retinal organoids for 80 days, to give several different types of eye cells time to mature. They then broke the organoids up into individual cells and cultured them together.
During the breaking up of the organoids, all existing synaptic connections were destroyed. However, 20 days after the researchers isolated the individual cells, all the cell types could once again produce proteins required to form new synapses.
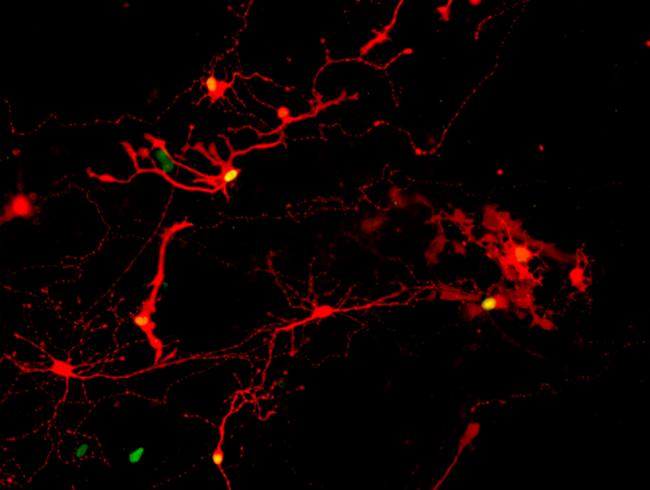
To track whether these isolated cells could form synaptic connections, the team cultured the cells along with a tracer based on the rabies virus. Rabies virus travels from nerve cell to nerve cell through synapses. By pairing a harmless version of the virus with fluorescent tracers, the team could track it through any new synaptic connections.
Using the tracer, the researchers found that the cells formed new synaptic connections. Several types of eye cells were found in the new neural circuits. Photoreceptors were the most common, followed by retinal ganglion cells.
“The last piece of the puzzle,” Gann says, “was to see if these [cells] had the ability to plug into, or shake hands with, other retinal cell types in order to communicate.” Now that this ability has been proven, the team will work to adapt the technology to treat human eye disorders.
—by Sharon Reynolds
Related Links
- Gene Therapy with Novel Protein Restores Vision in Mice
- Lab-Made Eye Cells Restore Vision in Mice
- Patch Replaces Damaged Retinal Cells
- Regenerating Light-Sensing Eye Cells in Mice
- Immune Cell Regeneration in Mouse Retina
- Regenerating Retinal Cells in Mice
- New Color Vision Pathway Unveiled
- Stem Cells Form Light-Sensitive 3-D Retinal Tissue
- Healthy Vision
- Tissue Engineering and Regenerative Medicine
References
Re-formation of synaptic connectivity in dissociated human stem cell-derived retinal organoid cultures. Ludwig AL, Mayerl SJ, Gao Y, Banghart M, Bacig C, Fernandez Zepeda MA, Zhao X, Gamm DM. Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2213418120. doi: 10.1073/pnas.2213418120. Epub 2023 Jan 4. PMID: 36598946.
Funding
NIH’s National Eye Institute (NEI), National Institute of Mental Health (NIMH), and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); Department of Defense; Fighting Blindness Canada; Research to Prevent Blindness; Retina Research Foundation; Emmett A. Humble Distinguished Directorship; McPherson ERI Sandra Lemke Trout Chair in Eye Research; Arthur and Nancy Nesbitt; University of Wisconsin-Madison School of Veterinary Medicine Dean's Office; UW-Madison Stem Cell and Regenerative Medicine Center; Foundation Fighting Blindness.


