Scientists find subtypes of senescence
May 13, 2025
Scientists find subtypes of senescence
At a Glance
- Researchers found that cells in skin that stop dividing but don’t die, called senescent cells, aren’t all the same, coming in three types with distinct treatment responses.
- The findings suggest it may be possible to develop therapies for removing harmful senescent cells while leaving others behind.
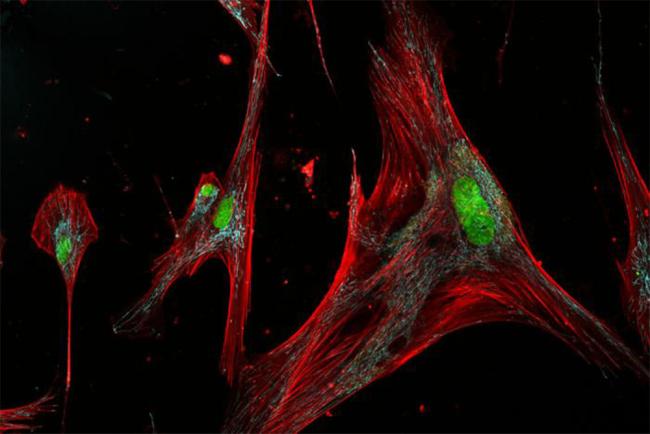
As we age, cells in our bodies become diseased, injured, and stressed. While many die and get removed by the immune system, others enter a state known as senescence. Senescent cells stop dividing and make inflammatory substances, among other changes. Researchers have suspected that treatments to remove these cells might be a key to healthier aging.
Past studies have hinted at different subtypes of senescent cells. NIH-funded researchers led by Dr. Jude Phillip at John Hopkins University wanted to take a closer look. To do it, they developed an integrated single-cell imaging and machine learning framework to identify and classify senescent, individual cells. They call their new system SenSCOUT (short for senescence subtype classifier based on observable unique phenotypes). They described the system and their findings in Science Advances on April 25, 2025.
The researchers focused their attention on skin fibroblasts, fibrous cells that support and connect tissues and organs in the body. They isolated fibroblasts from a relatively young donor, aged 23, and an older person, aged 85, and grew them in the lab. They then induced senescence in different ways and examined the cells’ visual features, what proteins they made, and what compounds they secreted. In total, the team examined more than 50,000 single cells.
When the scientists profiled the fibroblast cells, they found 11 different subtypes with distinct shapes. Three of those subtypes, which they call C7, C10, and C11, scored highly for senescence. Other subtypes had features of senescence, but it wasn’t clear if they, too, could be classified as senescent cells.
To learn more about how the different subtypes related to a person’s age, the researchers looked to samples from a group of 50 healthy donors, aged 20 to 89. The donors were participants in a long ongoing study of aging in the U.S. Again, the team found three senescent cell subtypes.
As expected, the number of senescent cells rose with a person’s age. However, the C10 subtype increased in numbers with aging more than the other two. Overall, the relationship between chronological age and how susceptible cells were to senescence was not straightforward.
The team also saw differences in tests of how the different subtypes responded to drugs designed to target and kill senescent cells. Known as senotherapeutics, such treatments hold promise for reversing or slowing aging. The C7 subtype responded more than the others to treatment with an experimental anti-aging combination that included dasatinib and quercetin.
The findings raise several questions. For instance, the researchers don’t yet know which of the senescent cell subtypes may be harmful. They aim to learn more about them in living skin and explore how they affect skin diseases and aging.
The tools the researchers developed can now be used to study senescent cells in various parts of the body. In addition to anti-aging, the findings may help lead to treatments to remove the buildup of senescent cells after cancer treatments. They may also suggest new approaches for other conditions in which senescent cells play a role.
“We hope, with some more development, our technology will be used to help predict which drugs might work well for targeting senescent cells that contribute to specific diseases,” Phillip says. “Eventually, the dream is to be able to provide more information in a clinical setting to help with individual diagnoses and boost health outcomes.”
—by Kendall K. Morgan, Ph.D.
Related Links
- Assessing Ways to Gauge Aging Status
- Research in Context: Can We Slow Aging?
- Tracking Organ Aging and Disease
- Drugs Treat Spinal Disc Degeneration in Mice
- Blood Protein Signatures Change Across Lifespan
- Senescent Cells Tied to Health and Longevity in Mice
- Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing (GESTALT)
References
Single-cell morphology encodes functional subtypes of senescence in aging human dermal fibroblasts. Kamat P, Macaluso N, Li Y, Agrawal A, Winston A, Pan L, Stewart T, Starich B, Milcik N, Min C, Wu PH, Walston J, Fan J, Phillip JM. Sci Adv. 2025 Apr 25;11(17):eads1875. doi: 10.1126/sciadv.ads1875. Epub 2025 Apr 25. PMID: 40279419.
Funding
NIH’s National Cancer Institute (NCI), National Institute on Aging (NIA), and National Institute of General Medical Sciences (NIGMS); American Federation for Aging Research; Johns Hopkins University; Salisbury Family Center for Innovative Medicine Human Aging Project; Whiting School of Engineering at Johns Hopkins University.


