Lizard tails give clues to cartilage formation
August 22, 2023
Lizard tails give clues to cartilage formation
At a Glance
- Scientists identified cells that play key roles in cartilage creation and the regrowth of severed tails in a small lizard.
- The findings add to our understanding of tissue regeneration and may point to new avenues for repairing damaged cartilage in humans.

Many lizards, such as the green anole, have the ability to detach and regrow their tails, which helps them escape the grasp of predators. But the new tail’s main structural component is made of cartilage rather than the bone that was in the original tail. The regrown lizard tail also includes tissues like muscle, nerves, and blood vessels.
Scientists have been studying the lizard’s unique ability to regenerate its tail in hopes that it can offer new insights into cartilage growth or limb repair in people. Most mammals, including humans, respond to tissue injury or limb loss by forming fibrous or scar tissues instead of activating a regenerative process. Human adults are not able to naturally heal or regrow damaged cartilage.
To learn more about cartilage creation, a research team led by Dr. Thomas Lozito at the University of Southern California examined cellular and molecular details of limb regeneration in the anole lizard. They used a technique called single-cell RNA sequencing to determine which types of cells were present at different time points after tail loss in lizards, and which types of genes were activated. Their results were reported in Nature Communications on August 10, 2023.
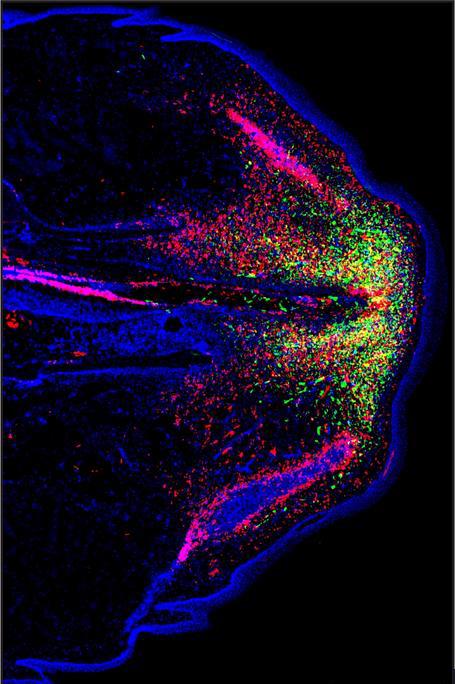
The scientists gained new insights into two types of cells, called fibroblasts and phagocytes, that are essential to forming new cartilage in the regrowing tail. Fibroblasts are a type of connective tissue cell. They make and secrete collagen and other proteins that help to maintain the structure of tissues. Analyses revealed different subsets of fibroblasts that played different roles in forming cartilage during the 28 days after tail loss.
Phagocytes are a type of immune cell that protects the body by gobbling up bacteria, foreign particles, and dead or dying cells. Phagocytes and other immune cells are known to congregate at injured sites. Tail regrowth involved distinct locations for different phagocytes. Factors secreted by certain phagocytes proved critical for signaling fibroblasts to build new cartilage. One particular type of phagocyte, called a septoclast, was especially important for regrowing lizard tails.
To clarify the role of septoclasts, the researchers isolated these cells from lizard tails and transferred the factors they secreted into lizards that had an amputated leg. Lizard legs, like mammal limbs, do not naturally regrow when injured. Rather, they tend to form fibrous and scar tissues, which inhibit regrowth. The scientists found that factors from septoclasts could suppress scarring in severed lizard limbs and enable formation of new cartilage.
The researchers also identified a well-known signaling pathway, called Hedgehog, that is crucial for cartilage formation. Lizards treated with a drug that blocks Hedgehog regrew tails that were normal length but lacked cartilage.
“Those two cell types working together laid the foundation for the beginning of the regenerative process,” Lozito says. “This represents an important step, because we need to understand the process in great detail before we can try to recreate it in mammals.”
—by Vicki Contie
Related Links
- 3D-Printed Scaffold Engineered to Grow Complex Tissues
- Human Skeletal Stem Cell Identified
- Fixing Flawed Body Parts: Engineering New Tissues and Organs
- Tissue Engineering and Regenerative Medicine
References
Single-cell analysis of lizard blastema fibroblasts reveals phagocyte-dependent activation of Hedgehog-responsive chondrogenesis. Vonk AC, Zhao X, Pan Z, Hudnall ML, Oakes CG, Lopez GA, Hasel-Kolossa SC, Kuncz AWC, Sengelmann SB, Gamble DJ, Lozito TP. Nat Commun. 2023 Aug 10;14(1):4489. doi: 10.1038/s41467-023-40206-z. PMID: 37563130.
Funding
NIH’s National Institute of General Medical Sciences (NIGMS) and National Cancer Institute (NCI).


