How mucus in the colon prevents inflammation and injury
November 3, 2020
How mucus in the colon prevents inflammation and injury
At a Glance
- A study in mice showed how mucus production throughout the colon keeps gut bacteria isolated from intestinal tissue and helps prevent inflammation.
- A better understanding of healthy mucus production could lead to strategies for treating inflammatory bowel disease and other gut disorders.
The gut microbiome consists of trillions of bacteria and other microorganisms that normally live in our intestines. They have many important functions, such as helping digest food and preventing dangerous bacteria from getting a foothold in the body.
Problems can result when the relationship between the microbiome and the digestive system becomes disrupted. Imbalances are thought to contribute to many gut disorders, including the inflammatory bowel diseases ulcerative colitis and Crohn’s disease.
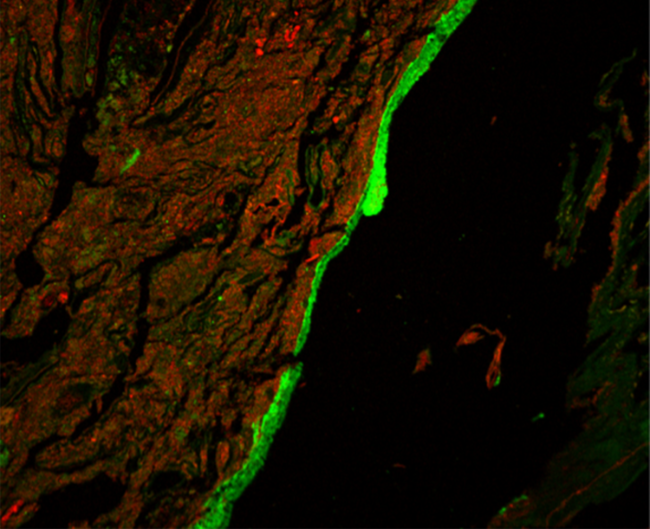
The large intestine, or colon, produces protective layers of mucus, a network of proteins rich in sugars added through a process called O-glycosylation. Previous studies suggested that this mucus plays a role in maintaining a healthy relationship between intestinal tissues and gut bacteria. But it has been hard to study mucus production in samples taken out of the intestines.
A research team led by Dr. Lijun Xia from the Oklahoma Medical Research Foundation developed an imaging method to visualize mucus production in the entire colons of mice. They examined O-glycosylated mucus production and its effects on gut bacteria in healthy mice and those with a disrupted microbiome.
The study was funded in part by NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of General Medical Sciences (NIGMS). Results were published on October 23, 2020, in Science.
The imaging showed that, contrary to current thinking, most gut mucus production happened in the proximal colon (the first and middle parts). Clumps of fecal matter and bacteria were coated with this mucus as they traveled through the colon. They then picked up a different type of mucus produced in the distal colon (the last part, closer to the rectum). Together, the two types of mucus encased the fecal pellets and largely kept bacteria from directly interacting with the tissue lining the colon.
When the team disrupted mucus O-glycosylation, this protective gut barrier broke down. Mice engineered to lack the sugar molecules necessary to make mucus in one or both parts of the colon could not encapsulate fecal pellets as effectively. These mice also had microbes living substantially closer to their gut tissue than mice with healthy mucus production. The O-glycosylated mucus affected the makeup and metabolic function of the encapsulated microbes as well.
Correspondingly, mice lacking these sugar molecules in the proximal colon were more vulnerable to developing inflammation and injury in the distal colon.
In mice without normal gut bacteria, mucus production in the proximal colon stalled. But when normal gut bacteria were restored or transplanted back into these mice, mucus production started up again. This suggests that the gut microbiome itself plays a role in promoting protective mucus production.
Rebalancing this mucus system may be a goal for future treatments of certain kinds of inflammation in the gut. The finding of mucus on fecal matter also opens new avenues to access and analyze intestinal mucus. The researchers found a similar mucus barrier on human and baboon fecal samples.
“Now that we better understand the role and origin of this mucus, we will study how we can supplement it or restore its production,” Xia says.
—by Sharon Reynolds
Related Links
- Gut Bacteria May Contribute to Abnormal Blood Vessel Formation
- Microbiome Influences Risk of Preterm Birth
- Gut Microbe Drives Autoimmunity
- Changing Gut Bacteria in Crohn’s Disease
- Diet Affects Autoinflammatory Disease Via Gut Microbes
- The Healthy Human Microbiome
- Your Microbes and You
- Gut Troubles


