Researchers create developmental map of mouse cochlea
June 9, 2020
Researchers create developmental map of mouse cochlea
At a Glance
- Scientists mapped how sensory cells develop in the mouse cochlea, a key sound-sensing structure in the inner ear.
- The findings could help in the development of therapies for some forms of hearing loss.
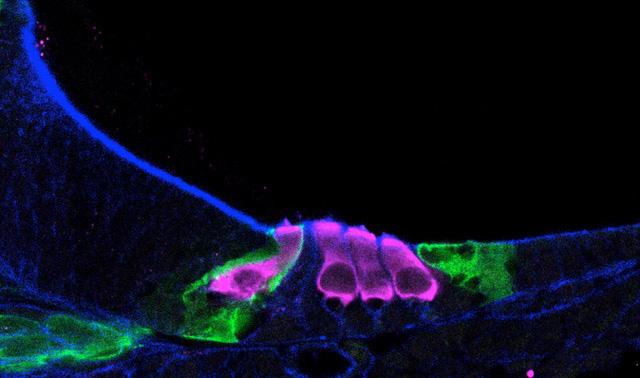
Hearing involves thousands of tiny hair cells inside the cochlea, a snail-shaped organ in the inner ear. Sound vibrations cause wispy bundles on top of each hair cell to sway. This movement sends electric signals through the auditory nerve to the brain, where the sound is interpreted.
In humans, hair cells can’t regenerate when they’re damaged. Loud sounds, disease, injury, and aging can all damage hair cells and result in permanent hearing loss.
Studying hair cells and the supporting cells that sustain them has been difficult. There are a limited number of these sensory cells, and they are closely packed within the cochlea in a region called the organ of Corti.
During development, a pool of progenitor cells gives rise to two kinds of hair cells, called inner and outer hair cells, as well as supporting cells. But the process that transforms progenitor cells into these unique cell types isn’t well understood.
A team led by Dr. Matthew W. Kelley of NIH’s National Institute on Deafness and Other Communication Disorders (NIDCD) has been studying how the cochlea develops. In previous work, they analyzed 301 cells from the mouse inner ear. In their new study, they analyzed the gene activity of 30,000 cells from the mouse cochlea to create a developmental map. Findings were published in Nature Communications on May 13, 2020.
The team used a method called single-cell RNA sequencing. This technique allows researchers to analyze the gene activity of single cells. Scientists can learn a lot about a cell from its pattern of active genes, which helps to define its function. Cells’ gene activity patterns can change during development or in response to the environment.
The researchers examined the gene activity profiles of mouse cochlear cells collected at four time points from the 14th day of embryonic development to the seventh day after birth. This enabled them to identify known cell types as well as ones that were previously unknown. It also allowed them to track the developmental pathways of the different cell types.
One gene of note that was active in the progenitors of outer hair cells early in embryonic development was Tgfbr1. Tgfbr1 has been linked to two conditions that can lead to hearing loss, Ehlers-Danlos syndrome and Loeys-Dietz syndrome. To understand Tgfbr1’s role, the researchers blocked the Tgfbr1 protein’s activity in mouse embryos. Several days later, the cochleae had fewer outer hair cells than normal. This result suggests that hearing loss in people with Tgfbr1 mutations could stem from impaired outer hair cell formation during development.
The researchers also found evidence that inner and outer hair cells diverge early in development, suggesting distinct pools of precursor cells. This and other insights into cochlear development could help those working to transform stem cells into replacements for damaged hair cells.
“Unlike many other types of cells in the body, the sensory cells that enable us to hear do not have the capacity to regenerate when they become damaged or diseased,” says NIDCD Director Dr. Debara L. Tucci. “By clarifying our understanding of how these cells are formed in the developing inner ear, this work is an important asset for scientists working on stem cell-based therapeutics that may treat or reverse some forms of inner ear hearing loss.”
Related Links
- Technique Treats Hereditary Deafness in Mice
- Protein Involved in Hearing Loss Recovery
- A Blueprint of Cell Development in the Inner Ear
- Hearing Different Frequencies
- Insights Into Inner Ear Repair
- Key Hearing Proteins Identified
- Hearing, Ear Infections, and Deafness
References
Characterization of the development of the mouse cochlear epithelium at the single cell level. Kolla L, Kelly MC, Mann ZF, Anaya-Rocha A, Ellis K, Lemons A, Palermo AT, So KS, Mays JC, Orvis J, Burns JC, Hertzano R, Driver EC, Kelley MW. Nat Commun. 2020 May 13;11(1):2389. doi: 10.1038/s41467-020-16113-y.PMID: 32404924
Funding
NIH’s National Institute on Deafness and Other Communication Disorders (NIDCD); King’s College London; Hearing Health Foundation.


