Scientists create a developing mouse embryo model from stem cells
September 13, 2022
Scientists create a developing mouse embryo model from stem cells
At a Glance
- Using mouse stem cells, researchers created a model embryo that mirrors brain and heart development.
- The model embryos could be used to study factors affecting embryonic development.
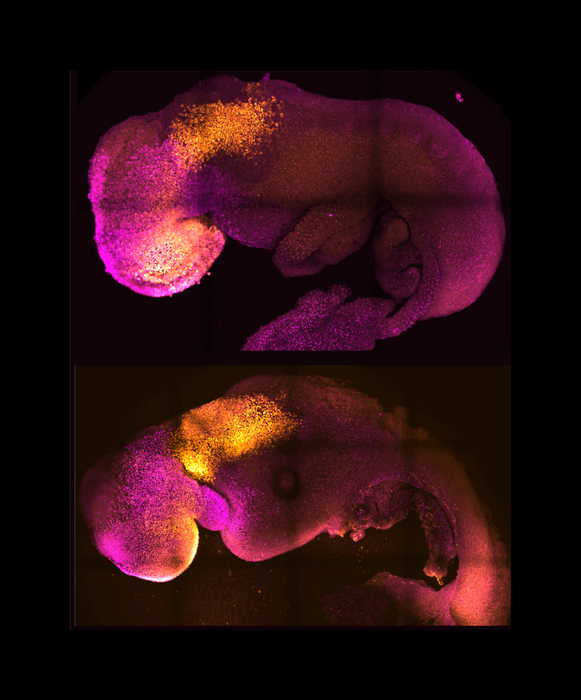
Embryonic stem cells from mammals can start to form embryo-like structures in the laboratory. But they don’t form all the structures that natural embryos do. That’s because certain developmental steps depend on interactions with other cell types outside the embryo. These include trophoblast and extraembryonic endoderm cells. The former develop into the placenta while the latter form the yolk sac that supplies blood and nourishment to the early embryo.
An NIH-funded research team, led by Dr. Magdalena Zernicka-Goetz at the University of Cambridge and the California Institute of Technology, has been trying to coax a stem cell-derived early embryo to develop further in the lab. To do so, they combined mouse embryonic stem cells with trophoblast and extraembryonic endoderm cells and cultured them in conditions designed to permit the development seen after implantation. The results of their efforts appeared in Nature on August 25, 2022.
The cultured cells self-organized into structures resembling natural mouse embryos. The team calls these ETiX-embryoids. These embryoids developed all the specific cell types and structures that natural embryos do during the first 8.5 days after fertilization, including the yolk sac surrounding the embryo. By day 7, they had headfolds at one end and a tail bud at the other. A neural tube ran along the axis between them. By day 8, the embryoids contained cell types seen in early organ development.
In natural embryos, paired blocks of cells called somites form along both sides of the neural tube. These develop into skeletal muscle, vertebrae, cartilage, and other structures. The researchers observed somites in the ETiX-embryoids on day 7. On day 8, they observed a beating structure resembling a primitive heart. The beating structure expressed genes required for cardiac development. The team also saw a gut tube corresponding to that seen in the developing guts of natural embryos.
Primordial germ cells, the precursors of sperm and egg cells, were seen in the embryoids as early as day 6. A membrane resembling the yolk sac surrounded the developing embryoids. This yolk sac contained areas from which blood vessels develop.
The researchers examined gene activity, or expression, patterns throughout the embryoids. The tissue-specific patterns matched those seen in natural embryos. Gene expression in the headfolds on day 8 indicated the presence of defined forebrain and midbrain regions. Of note, removing a gene essential for neural tube development had the same effect in the embryoids and natural embryos.
These findings show that a combination of stem cells can mimic the early stages of embryonic development in mammals. This could be a useful model system for studying factors affecting embryonic development. For example, it could help researchers understand why some embryos fail to develop into healthy pregnancies.
“This period of human life is so mysterious, so to be able to see how it happens in a dish—to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening—is quite special,” Zernicka-Goetz says.
Related Links
- How a Marine Animal Makes Unlimited Eggs and Sperm
- Insights Into How Female Embryos Develop
- Developing Insulin-Producing Cells to Treat Diabetes
- Stem Cells Form Light-Sensitive 3-D Retinal Tissue
- Stem Cell Therapy Rebuilds Heart Muscle in Primates
- NIH Stem Cell Information
References
Synthetic embryos complete gastrulation to neurulation and organogenesis. Amadei G, Handford CE, Qiu C, De Jonghe J, Greenfeld H, Tran M, Martin BK, Chen DY, Aguilera-Castrejon A, Hanna JH, Elowitz M, Hollfelder F, Shendure J, Glover DM, Zernicka-Goetz M. Nature. 2022 Aug 25. doi: 10.1038/s41586-022-05246-3. Online ahead of print. PMID: 36007540.
Funding
NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Human Genome Research Institute (NHGRI); European Research Council; Wellcome Trust; Silicon Valley Community Foundation; Weston Havens Foundation; Centre for Trophoblast Research; Biotechnology and Biological Sciences Research Council (BBSRC); A. G. Leventis Foundation; Paul G. Allen Frontiers Group; Howard Hughes Medical Institute; California Institute of Technology.


