Potent neutralizing antibodies target new regions of coronavirus spike
August 4, 2020
Potent neutralizing antibodies target new regions of coronavirus spike
At a Glance
- Researchers identified some of the most potent and diverse antibodies discovered to date that neutralize SARS-CoV-2, targeting multiple regions on the viral spike.
- Potent antibodies could be produced in the lab and given to people to prevent or treat coronavirus infection.
Scientists are racing to develop approaches to protect against SARS-CoV-2, the virus that causes COVID-19. Several vaccine candidates are now in clinical trials. Another possible approach involves antibodies, proteins made by the immune system to fight infection. Antibodies work to neutralize viruses by binding to their surface and blocking entry into a person’s cells.
Antibodies that neutralize SARS-CoV-2 could be produced in a lab and given to people to treat infection. These lab-made antibodies, called monoclonal antibodies, could also serve as a prevention option until a vaccine is available. Vaccines prompt the immune system to make antibodies against a virus. Monoclonal antibodies are delivered directly to the body, through injection or intravenous infusions, and circulate in the blood.
Monoclonal antibodies are currently used to treat a variety of conditions, including rheumatoid arthritis, Crohn’s disease, and cancer. They may be the only option for older adults and others who can’t develop or maintain an adequate immune response after vaccination. But researchers must first identify powerful antibodies to neutralize SARS-CoV-2.
To this end, researchers led by Drs. Yaoxing Huang, Lawrence Shapiro, and David D. Ho of Columbia University isolated and tested antibodies from hospitalized COVID-19 patients. The team included scientists from NIH’s National Institute of Allergy and Infectious Diseases (NIAID). The study was funded in part by NIH’s National Institute of General Medical Sciences (NIGMS) and Common Fund. Findings appeared in Nature on July 15, 2020.
The researchers screened hospitalized COVID-19 patients and chose five with the highest levels of neutralizing activity in their blood plasma. They then isolated immune cells from their blood called memory B cells. These cells, created during an infection, can last for years in the body and be quickly activated to produce antibodies when a virus or other microbe is encountered again.
The team isolated memory B cells specific to the coronavirus spike protein and used them to create 252 monoclonal antibodies in the lab. These antibodies were then screened for their ability to neutralize the virus.
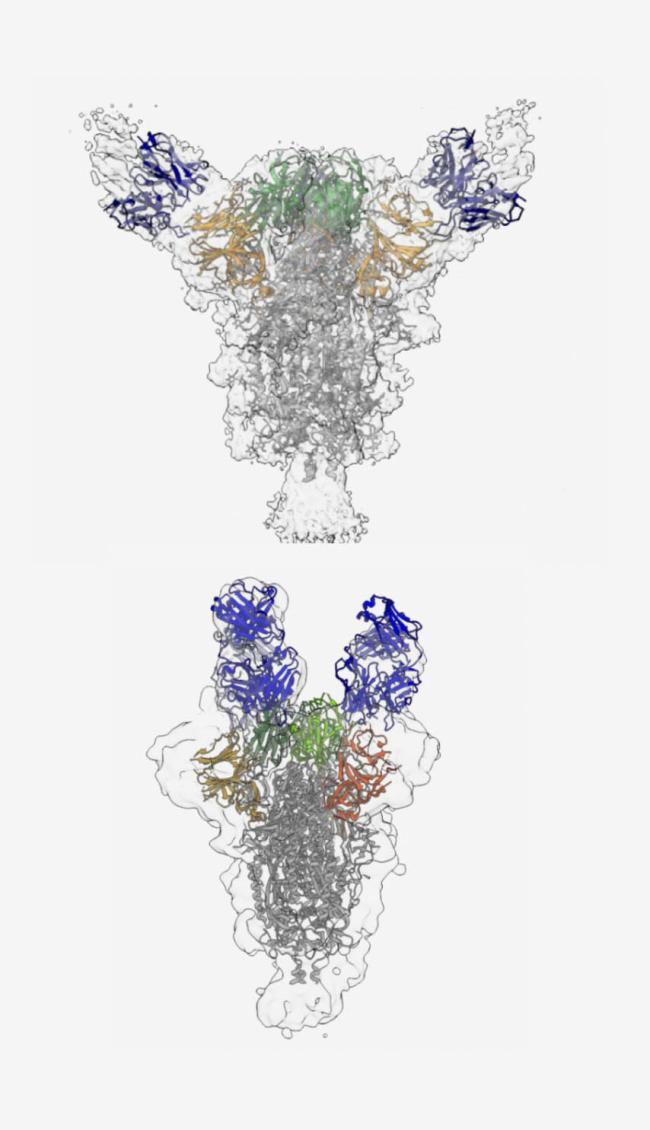
The team identified 19 antibodies that potently neutralized SARS-CoV-2. Nine bound to the receptor binding domain (RBD), a vital part of the spike protein that allows the virus to dock onto human cells. Eight targeted a part of the upper spike protein called the N-terminal region. Two antibodies bound to other nearby regions. Previous studies have identified antibodies that target the RBD. This reveals a more diverse variety of antibodies than previously reported.
Using a technique called cryo-electron microscopy, or cryo-EM, the researchers gained insight into where the potent antibodies bound to the surface of the viral spike. This was the first publication showing the structure of a neutralizing antibody bound to the N-terminal region. The team also confirmed that one of the purified antibodies provided strong protection against SARS-CoV-2 infection in hamsters.
“These findings show which sites on the viral spike are most vulnerable,” Ho says. Based on these results, several of the monoclonal antibodies appear promising for further development, and the team is now planning additional studies in other animals and people.
—by Erin Bryant
Related Links
- Experimental Coronavirus Vaccine is Safe and Produces Immune Response
- Potent Antibodies Found in People Recovered from COVID-19
- Unique Genomic Features of Fatal Coronaviruses
- Cancer Drug May Reduce Symptoms of Severe COVID-19
- Early Results Show Benefit of Remdesivir for COVID-19
- Llama Antibody Engineered to Block Coronavirus
- Microneedle Coronavirus Vaccine Triggers Immune Response in Mice
- Study Suggests New Coronavirus May Remain on Surfaces for Days
- Novel Coronavirus Structure Reveals Targets for Vaccines and Treatments
- Coronavirus (COVID-19)
- Coronavirus Prevention Network


