Coronaviruses hijack lysosomes to exit cells
November 10, 2020
Coronaviruses hijack lysosomes to exit cells
At a Glance
- Researchers discovered that coronaviruses hijack lysosomes, the cell’s trash disposal system, to exit cells and spread through the body.
- The study may provide insight into stopping transmission of the virus that causes COVID-19.
COVID-19 cases are surging worldwide. With U.S. deaths nearing 225,000, scientists are working to better understand how the virus that causes the disease infects cells and spreads through the body. SARS-CoV-2, like most viruses, enters and infects cells, and then uses the cell’s protein-making machinery to make multiple copies of itself. It must then escape the cell. While researchers have learned a great deal about how the virus infects cells, they have only a limited understanding of how it exits them.
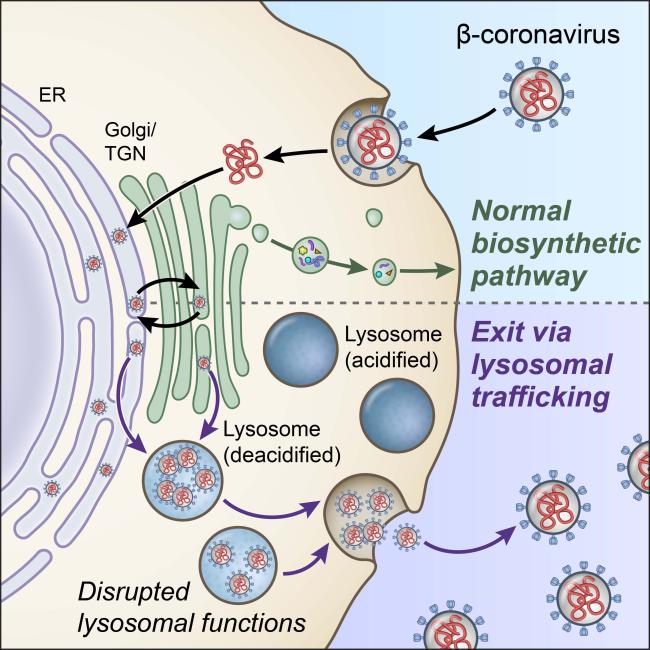
Most viruses—including hepatitis C, Dengue, and West Nile—exit through the “biosynthetic secretory” pathway. That’s the central pathway that cells use to transport hormones, growth factors, and other materials to their surrounding environment.
A research team led by Drs. Nihal Altan-Bonnet and Sourish Ghosh at NIH’s National Heart, Lung, and Blood Institute (NHLBI) and Dr. Gregoire Altan-Bonnet at NIH’s National Cancer Institute (NCI) investigated whether coronaviruses also use this pathway to exit cells. For their experiments, they used a related coronavirus called MHV as well as SARS-COV-2. The study, which was funded by several NIH Institutes, was published on October 27, 2020 in Cell.
The researchers chemically blocked the biosynthetic secretory pathway in MHV-infected cells. Using microscopic imaging and virus-specific markers, they discovered that the viruses still left cells with this pathway blocked.
Further experiments revealed that the viruses instead exited infected cells through the lysosome, an organelle that serves as the cells’ trash disposal system. Normally, the lysosome’s acidic environment helps destroy viruses and other pathogens before leaving cells. SARS-CoV-2 was also found in the lysosomes of infected cells.
The scientists discovered that lysosomes were de-acidified in coronavirus-infected cells. This significantly weakened the activity of their destructive enzymes. As a result, the coronaviruses remained intact and ready to infect other cells when they exited.
Certain immune system cells rely on lysosomes to degrade proteins into short pieces that help trigger other immune cells to respond. The researchers found that the coronavirus deactivated this disease-fighting function of the lysosome. De-acidification of the lysosome may alter other immune system functions as well. The findings could help explain some of the immune system abnormalities seen with COVID-19 patients.
“These coronaviruses are very sneaky,” Dr. Nihal Altan-Bonnet says. “They’re using these lysosomes to get out, but they’re also disrupting the lysosome so it can’t do its job or function.”
Now that this mechanism has been identified, researchers may be able to find ways to disrupt the pathway. The authors identified one experimental compound that potently blocked coronaviruses from getting out of cells. However, more studies are needed to determine whether this drug or others that block the lysosome pathway could be used to combat COVID-19.
Related Links
- Final Report Confirms Remdesivir Benefits for COVID-19
- SARS-CoV-2 May Use Key Carbohydrate to Infect Cells
- Computer-Designed Proteins May Protect Against Coronavirus
- Immune Cells for Common Cold May Recognize SARS-CoV-2
- Potent Neutralizing Antibodies Target New Regions of Coronavirus Spike
- Experimental Coronavirus Vaccine is Safe and Produces Immune Response
- Potent Antibodies Found in People Recovered from COVID-19
- Cancer drug may reduce symptoms of severe COVID-19
- Coronavirus Prevention Network
References
β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Ghosh S, Dellibovi-Ragheb TA, Kerviel A, Pak E, Qiu Q, Fisher M, Takvorian PM, Bleck C, Hsu VW, Fehr AR, Perlman S, Achar SR, Straus MR, Whittaker GR, de Haan CAM, Kehrl J, Altan-Bonnet G, Altan-Bonnet N. Cell. 2020 Oct 27:S0092-8674(20)31446-X. doi: 10.1016/j.cell.2020.10.039. Online ahead of print. PMID: 33157038.
Funding
NIH’S National Heart, Lung, and Blood Institute (NHLBI), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Neurological Disorders and Stroke (NINDS), National Institute of General Medical Sciences (NIGMS), and National Cancer Institute (NCI).


