Gaps allow substances to move in and out of the brain
February 27, 2024
Gaps allow substances to move in and out of the brain
At a Glance
- Gaps around blood vessels that cross the layers of tissues protecting the brain allow certain cells and molecules to move between the brain and the body.
- Better understanding of these structures could have implications for diseases in which waste doesn’t drain from the brain, or inflammatory cells can get in.
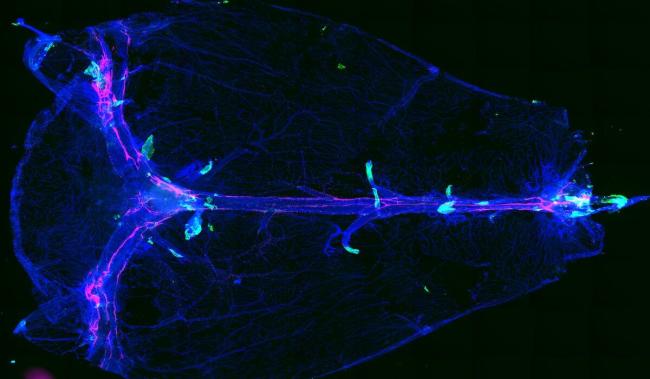
The human brain is both vital and vulnerable. Accordingly, it’s wrapped in many protective structures, from the skull itself to the blood-brain barrier, a tightly packed mix of cells that stops many molecules from entering from the bloodstream. Other protective barriers include the meninges, the layers of tissue that cover and protect the brain and spinal cord.
A tight layer of cells called the arachnoid barrier lies between the inner layers of the meninges and the outer layer, called the dura mater, which has more connections with the rest of the body. But the arachnoid barrier is not absolute. Immune cells are known to pass from the body through the meninges and into the brain, in part to protect against infection. And waste products produced by the brain can flow out to the dura mater for disposal. How exactly this movement happens across the arachnoid barrier has been unclear.
A new study led by Dr. Jonathan Kipnis of Washington University in St. Louis, in collaboration with Dr. Daniel Reich of NIH, aimed to understand the gates between the arachnoid barrier and dura mater. The researchers studied mice and people to track the flow of compounds and immune cells across the arachnoid barrier. Their results appeared on February 7, 2024, in Nature.
When examining different cell types in the layers of the mouse brain, the team noted that arachnoid barrier cells were found around blood vessels called bridging veins. These veins punch through the arachnoid barrier. The spaces around them were thought to be sealed tightly, like caulking around a pipe. However, the researchers found that the arachnoid barrier cells formed leaky cuffs around the bridging veins. They called these tiny openings arachnoid cuff exit (ACE) points.
Using high-powered microscopy and fluorescent tracers, the team observed molecules crossing from the inner layers of the meninges into the dura mater through these ACE points in live mice. Once these molecules reached the dura mater, they had direct access to the regions where waste drains out of the brain.
Correspondingly, the team found that molecules could also flow the other way across ACE points, from the dura mater across the arachnoid barrier and into the brain. In a mouse model of brain inflammation, the team observed changes in the ACE points that facilitated a harmful excess of immune cells to flow across the meninges.
The researchers next looked in people, using MRI imaging of a tracer commonly used in medical procedures. They observed the tracer rapidly spread from the dura mater to around the bridging veins in a pattern very similar to that seen in mice.
In older mice, drainage from the brain at the ACE points appeared slower than in younger brains. In older people, there was more leakage of the tracer into the cerebrospinal fluid. “This might point to a slow breakdown of the ACE points over the course of aging,” Reich notes. “This could be consequential in that the brain and immune system can now interact in ways that they’re not supposed to. Alternatively, the changes could be part of a protective mechanism that kicks in as we age.”
These results could have implications for Alzheimer’s disease and related conditions, which have been linked to the buildup of waste products in the brain. The findings could also lead to insights into how cancer cells and inflammatory immune cells enter the brain. More research is needed to understand whether the flow through ACE points could be manipulated therapeutically.
Related Links
- Immune Cells Control Waste Clearance in the Brain
- Boosting Brain’s Waste Removal System Could Improve Alzheimer’s Outcomes
- Alzheimer’s Gene Contributes to Blood-Brain Barrier Breakdown
- How Cancer Vesicles Breach the Blood-Brain Barrier
- Blood-Brain Barrier Test May Predict Dementia
- Impaired Brain Drainage in Aging and Alzheimer’s
- Brain Cleaning System Uses Lymphatic Vessels
- Boosting Brain’s Waste Disposal System May Slow Neurodegenerative Diseases
- Lymphatic Vessels Discovered in Central Nervous System
- Alzheimer's and Dementia
- Dementias
References
Identification of direct connections between the dura and the brain. Smyth LCD, Xu D, Okar SV, Dykstra T, Rustenhoven J, Papadopoulos Z, Bhasiin K, Kim MW, Drieu A, Mamuladze T, Blackburn S, Gu X, Gaitán MI, Nair G, Storck SE, Du S, White MA, Bayguinov P, Smirnov I, Dikranian K, Reich DS, Kipnis J. Nature. 2024 Feb 7. doi: 10.1038/s41586-023-06993-7. Online ahead of print. PMID: 38326613
Funding
NIH’s National Institute of Neurological Disorders and Stroke (NINDS) and National Institute on Aging (NIA); Cure Alzheimer’s Fund BEE Consortium; National MS Society.


